"Medical equipment" is a broad umbrella term that covers a wide variety of instruments and equipment, such as Band-Aids, dental floss, blood pressure cuffs, defibrillators, MRI scanners, and more. Medical device design is an important part of the field of mechanical engineering.
The medical device development process is no different than any other device: design, prototype, test and repeat. However, medical devices have stricter material requirements. Due to testing and clinical trial requirements, many medical device prototypes require biocompatible or sterilizable materials.
 1. Biocompatible materials
1. Biocompatible materials
For plastics, the most stringent requirement is the USP Class 6 test. The USP Level 6 test involves three in vivo bioreactivity assessments in animals, including:
• Acute Systemic Toxicity Test: This test measures the irritating effect of oral, dermal and inhaled samples.
• Intradermal test: This test measures the irritating effect of a sample in contact with living subdermal tissue.
• Implantation test: This test measures the stimulatory effect of intramuscular implantation of samples into test animals over five days.
3D printing can produce almost any geometry, which is useful for rapid iteration of complex designs. CNC machining is suitable for prototyping and end-use production of medical device parts. There are more materials to choose from, and the materials are stronger. However, the design requires more attention to ensure machinability.
The following materials are USP Class 6 Test Certified: POM, PP, PEI, PEEK, PSU, PPSU
If you are making early stage prototypes that will not be used in experiments or clinical trials, consider using non-certified plastics. You get the same mechanical performance without paying more. POM 150 is an excellent material for early prototyping.
CNC machining can also produce biocompatible metal parts. There are three common implant grade options:
• Stainless steel 316L
• Titanium grade 5, also known as Ti6Al4V or Ti 6-4
• Cobalt-chromium alloy (CoCr)

Stainless steel 316L is the most commonly used of the three materials. Titanium has a better weight-to-strength ratio, but is much more expensive. CoCr is mainly used in orthopedic implants. We recommend that you use the SS 316L for prototyping as you refine your design, then use the more expensive material as your design is more mature.
2. Sterilizable materials
Any reusable medical device that may come into contact with blood or body fluids must be sterilizable. Therefore, most medical devices used in medical facilities are made of sterilizable materials. There are many sterilization methods: heat (dry heat or autoclave/steam), pressure, chemicals, irradiation, etc.
Chemicals and irradiation are the preferred methods of plastic sterilization because heat can break down plastic. Here is a chart outlining the compatibility of many plastics with different sterilization methods. Autoclave and dry heat are common methods of metal sterilization.
All of the USP Class VI certified materials we mentioned earlier are sterilizable, as are implant-grade metals. Likewise, CNC machining offers the largest selection of sterilizable materials.
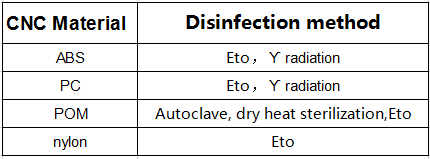
If the medical device is used for patient disinfection at home, the material should also be compatible with disinfection chemicals such as bleach, ethanol, isopropyl alcohol, iodine, and hydrogen peroxide. ABS and POM are the most chemically resistant plastics.
3. When to use medical grade materials
When building prototypes for experiments or clinical trials, be sure to use medical-grade materials. However, for early molding and assembly prototypes, using common materials can save you a lot of money.
----------------------------------------END----------------------------------------------



